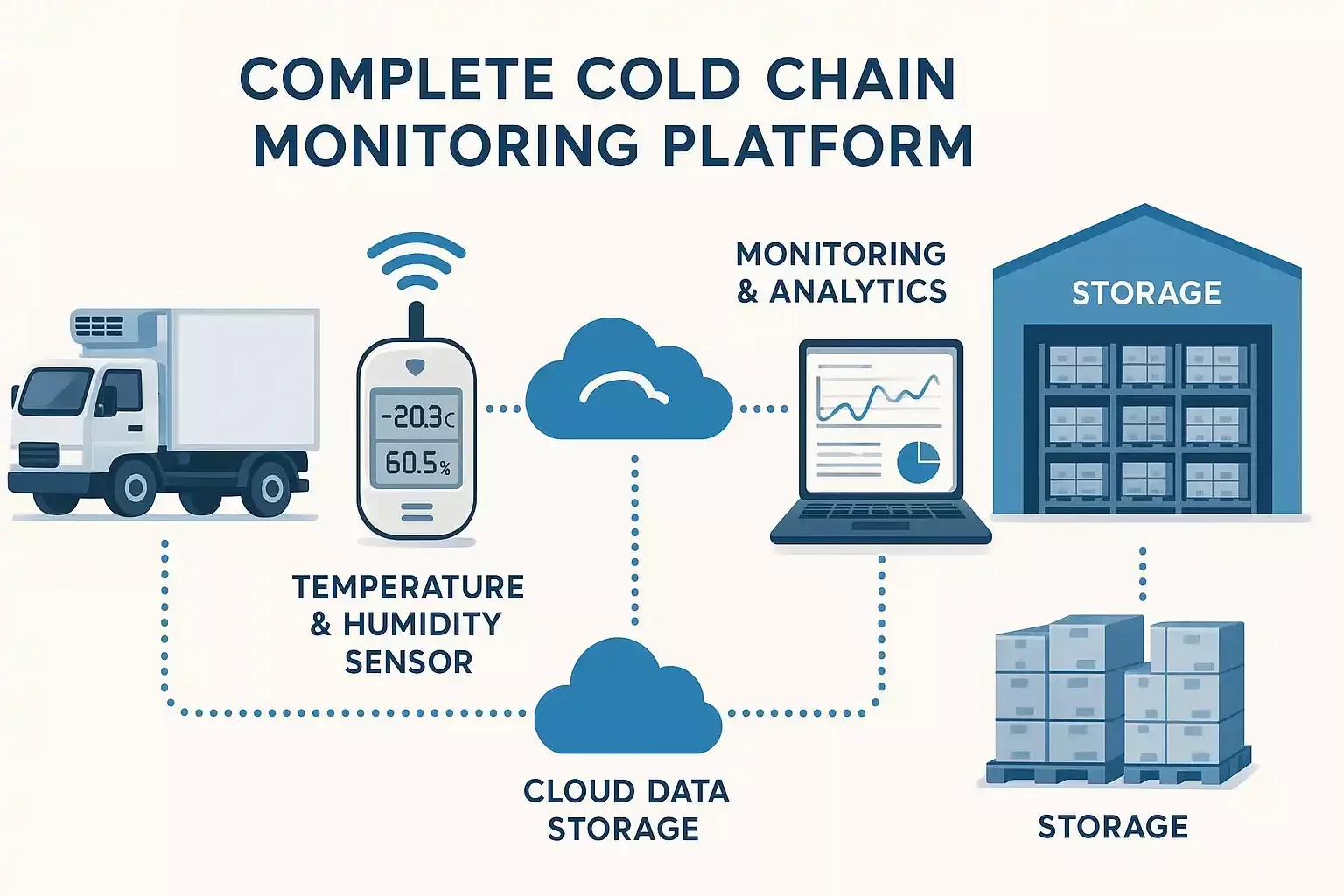
Audit-Ready Cold Chain Monitoring for Pharma, Food & Logistics.
Data integrity is non-negotiable. Seemoto transforms environmental monitoring from a manual burden into an automated compliance asset. Our system is engineered to meet the strictest global standards, ensuring you are always ready for an inspection, whether by the FDA, EU authorities, or your own customers.
Why Compliance Matters
Quick facts:

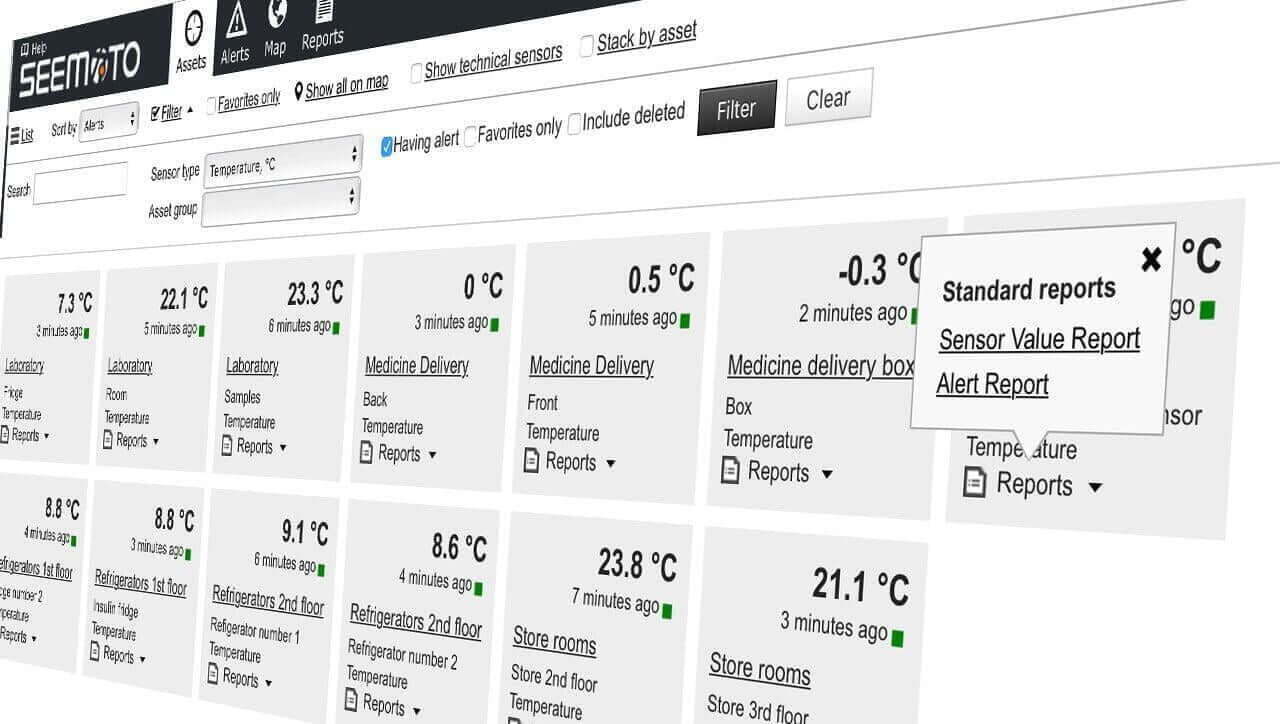
Standards at a Glance
| Standard | Key Focus | Seemoto |
|---|---|---|
| GDP | Pharma Supply Chain Integrity | End-to-End Traceability: Automated deviation management and continuous logging for storage & transport, fully aligned with EU GDP guidelines. |
| GMP | Production Quality Assurance | Environmental Control: Precision monitoring for cleanrooms and production areas to ensure strict manufacturing compliance. |
| EN12830 | Sensor Accuracy & Reliability | Class 1 Certified: Robust, waterproof (IP68) sensors validated for temperatures from -196°C to +150°C. |
| HACCP | Digital Data Integrity | Audit-Proof Records: Secure, unalterable electronic records with full audit trails, role-based access, and digital signatures. |
| FDA 21 CFR Part 11 | Food Safety Risk Control | CCP Automation: Replaces manual logs with automated Critical Control Point monitoring and instant hazard alerts |

Good Distribution Practice
Ensure product integrity across the entire supply chain. Seemoto provides continuous, validated monitoring for storage and transport, fully aligning with EU GDP guidelines (2013/C 343/01) for temperature-sensitive pharmaceuticals. Protect your license with automated deviation reports and route validation.

Good Manufacturing Practice
Maintain strict environmental control in production areas and cleanrooms. Seemoto delivers the validated data integrity and real-time deviation management required for GMP-regulated manufacturing. Seamlessly integrate environmental data with your existing quality systems.

EN12830 Standard
Rely on certified accuracy. All Seemoto TS and THS sensors are EN12830 Class 1 certified, ensuring precision for the entire cold chain. From -196°C deep freezers to +150°C ovens, our IP68-rated hardware provides the robust, independent verification auditors demand.

HACCP Food Safety
Automate your food safety plan. Replace manual clipboard checks with wireless monitoring to control Critical Control Points (CCPs) automatically. Instant hazard alerts prevent spoilage, while digital history logs ensure you pass ISO 22000 and health inspection audits effortlessly.
FDA 21 CFR Part 11
Secure your digital records. Seemoto provides the essential technical controls for FDA compliance: role-based access, electronic signatures, and unalterable, time-stamped audit trails. We ensure your data is authentic, retrievable, and ready for inspection at any moment.